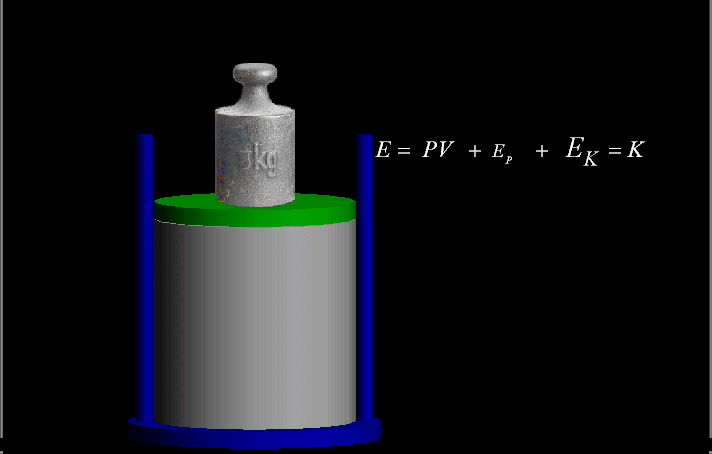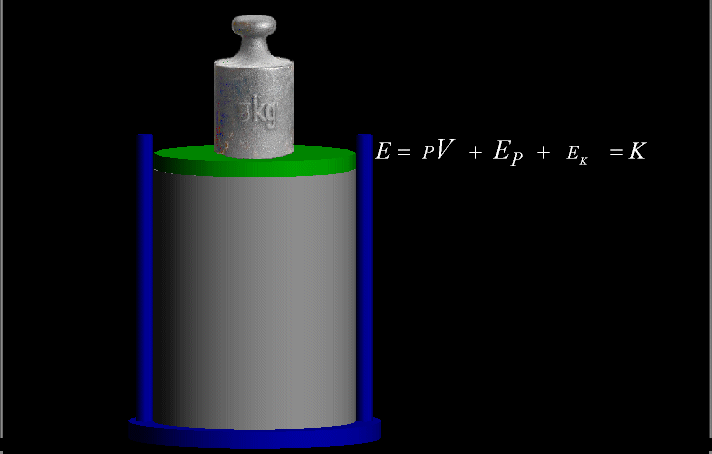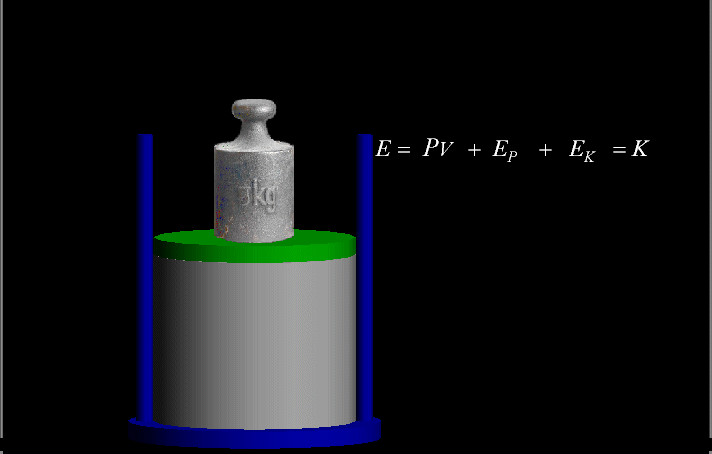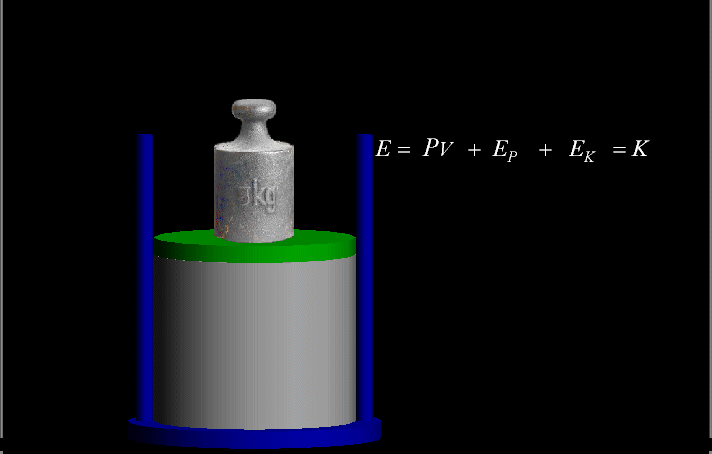
Your riches are corrupted, and your garments are motheaten.
Your gold and silver is cankered; and the rust of them shall be a witness against you,
and shall eat your flesh as it were fire. Ye have heaped treasure together for the last days.
Behold, the hire of the labourers ...
Your riches are corrupted, and your garments are motheaten.
Your gold and silver is cankered; and the rust of them shall be a witness against you,
and shall eat your flesh as it were fire. Ye have heaped treasure together for the last days.
Behold, the hire of the labourers ...
James 5:2-4